Introduction
Liposomes are spherical vesicles consisting of one or more concentric phospholipid bilayers enclosing an aqueous core. Nontoxic and biodegradable liposomes are useful tools to package both hydrophilic and hydrophobic biomolecules (including therapeutic agents) that may be further incorporated into target cells to elicit functional changes. Extensive research is being conducted using these nanocarriers in diverse areas including the delivery of anti-cancer, anti-fungal, anti-inflammatory drugs and therapeutic genes and proteins. Clinical trials have extensively shown that liposomes are pharmacologically and pharmocokinetically more efficient than drug-alone formulations and products including Doxil®, Ambisome® or DepoPur™ have been approved for clinical use.
The following labmethod covers the materials, equipment and a step-by-step protocol to produce liposomes composed by phosphatidylcholine (main lipid in vesicle formation), cholesterol (to enhance liposome stability and modulating membrane fluidity) and stearylamine (to confer a positive charge that will facilitate liposome-cell fusion events). Such liposomes have demonstrated major packaging capacity for incorporating proteins extracts used in mesenchymal stem cells reprogramming towards insulin-producing cells as new cell-based therapies for the treatment of type 1 diabetes.
Materials
- Egg yolk phosphatidylcholine (EYPC)
- Cholesterol (Ch)
- Stearylamine (SA)
- Methanol
- Chloroform
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
- Sodium chloride (NaCl)
- Ethylenediaminetetraacetic acid (EDTA)
- Nitrogen source
- Sephadex G-75
- Chromatography column
- 1 µm poresize membranes
- 2 ml microcentrifuge tubes
- 5 ml microcentrifuge tubes
- Parafilm
- Gauges
- Serological pipettes
- Tips for micropipettes
- Glass Pasteur pipettes
Equipment required
- Precision balance
- Pure nitrogen gas source
- Vacuum desiccator
- Fume hood
- HPLC UV/VIS detector
- Thermoblock
- LiposoFast
- Micropipettes
- Pipetor
- Fridge freezer
Methods
- Prepare a 7:2:1 molar ratio mixture of egg yolk phosphatidylcholine (EYPC): cholesterol (Ch): stearylamine (SA) lipids in 1:1 methanol-chloroform.
- Dry the mixture using an oxygen free-nitrogen flux and leave in a vacuum jar for 3 h for a complete drying.
- Rehydrate the resulting lipid film with the component/s to be encapsulated (protein extracts or DNA for cell reprogramming, specific drugs for therapeutic applications, etc.)
- Incubate the mixture in agitation at 35°C, vortexing samples periodically during 1 h in order to trap the component of interest into the multilamelar vesicles (MLVs)
- For final liposome preparation (large unilamelar vesicles: LUVs), disrupt MLVs under several free-thaw cycles during 1 h
- Extrude the lipid vesicle suspension through a 0.1 µm pore size membrane using a LiposoFast (25 times) in order to obtain homogeneous 100 nM LUVs.
- Separate LUV suspension from possible non-encapsulated material via size extrusion chromatography using Sephadex G-75 in the stationary phase and 10 mM HEPES, 150 mM NaCl and 0.1 mM EDTA as the mobile phase.
- Absorbance rising (260-590 nm) registered by an UV-light coupled detector indicates elution of the liposome fraction allowing collection of LUVs which can be stored for approximately 48 h without degradation.

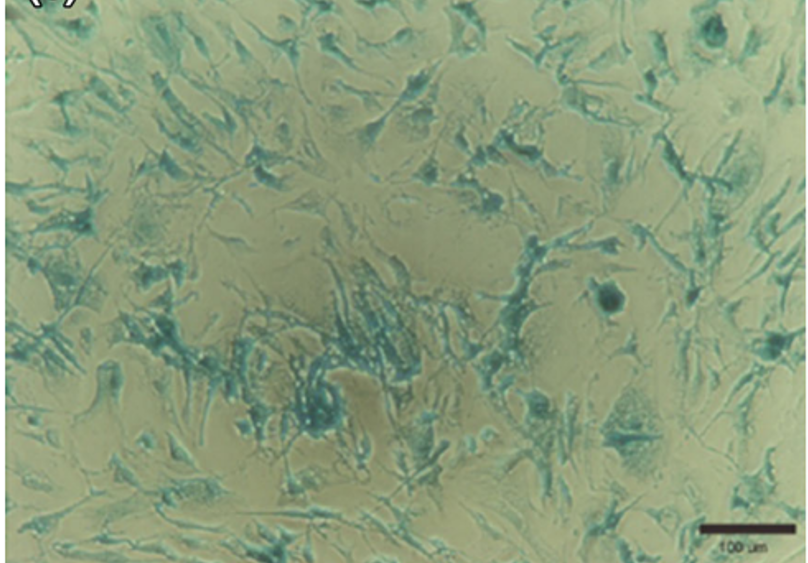
Micrograph of X-galactosidase staining of human mesenchymal stem cells incubated for 8 h with β-galactosidase packaged into liposomes as positive control
Reference:
Campillo N, Arribas MI, Vicente-Salar N, Catania A, Ramírez-Domínguez M, Reig JA, Domínguez-Bendala J, Micol V, Roche E. Transient alteration of gene expression in adipose-derived stem cells using liposomal-driven protein extracts. Cell Mol Bioeng, 2014; 7(1):145-154.
| Number | Category | Product | Amount |
|---|---|---|---|
| 1 | - | Egg yolk phosphatidylcholine | 1 |
| 2 | - | Cholesterol | 1 |
| 3 | - | Stearylamine | 1 |
| 4 | - | Methanol | 1 |
| 5 | - | Chloroform | 1 |
| 6 | - | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | 1 |
| 7 | - | Sodium chloride (NaCl) | 1 |
| 8 | - | Ethylenediaminetetraacetic acid (EDTA) | 1 |
| 9 | - | Sephadex G-75 | 1 |
| 10 | - | Chromatography column | 1 |
| 11 | - | 0.1 µm pore size membranes | 1 |
| 12 | - | 2 ml microcentrifuge tubes | 1 |
| 13 | - | 1.5 ml microcentrifuge tubes | 1 |
| 14 | - | Parafilm | 1 |
| 15 | - | Gauges | 1 |
| 16 | - | Serological pipettes | 1 |
| 17 | - | Tips for micropipettes | 1 |
| 18 | - | Glass Pasteur pipettes | 1 |